How to Determine Which Wave Function to Use
If one specifies initial conditions such that. To solve for the number of radial nodes the following simple equation can be used.
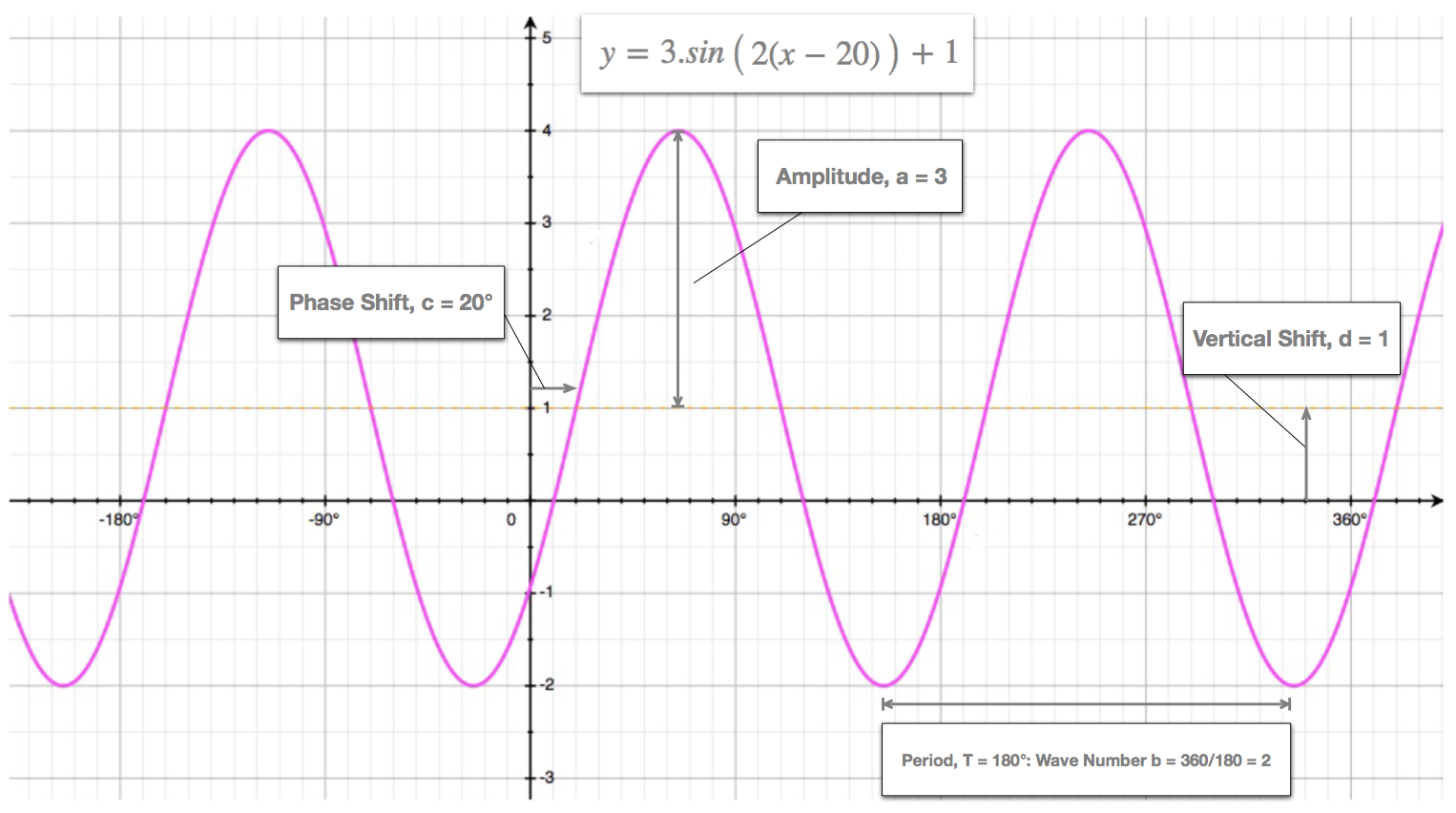
Transformed Cosine Sine Curves Wave Function
Where A is the amplitude of the wave function and is its wave number.
. Now we can rewrite this in another form using a trigonometric identity. Solution The wave function of the ball can be written. First loop over various momenta and get eigenvectors and eigenvalues.
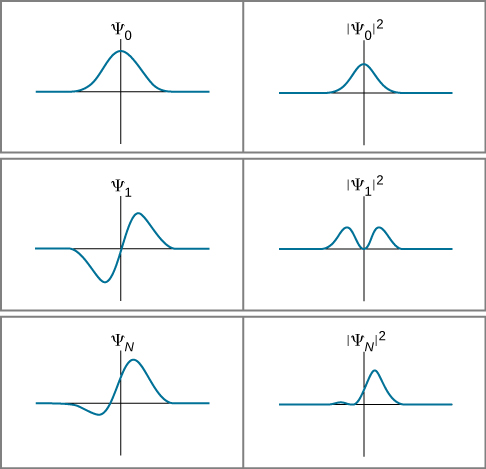
To obtain the probability density we calculate the square of the wave function. This will be automatically true for any valid mathematical function since this is a condition on functions from mathematical analysis. We can certainly make a measurement and determine the position of a particle at a particular time but then we loose the information about its energy.
We could also try to learn from the wave function the position of the particle. In the Probability Illustrator the value of the wave function is displayed next to an arrow that is just below the wave function graph. Ezt A coskz t Even more useful form for the solution E z t A kz t A kz t cos cos sin sin Thus our solution to the wave equation becomes.
It is possible to have a wave function that travels both to the left and to the right such as. Radial Nodes n - 1 - ℓ The n accounts for the total amount of nodes present. Is the general solution of the wave equation and thus carries no particular direction.
Cosz y cosz cosy sinz siny where. S i n 3 x We can see from the equation y 2. Cosxy cosx cosy sinx siny With this identity our solution becomes.
T θ Use the trigonometric identity. You square that value. It just gives you the probability and technically the square of it gives you the probability of finding the electron somewhere.
ν 1 L f v. How many nodes are in a circuit. It is inversely proportional to the functions period T.
To calculate the spatial wavenumber ν noting that L lambda means wavelength f means frequency and v means the speed of the wave. If your quantum physics instructor asks you to find the wave function of a hydrogen atom you can start with the radial Schrödinger equation R nl r which tells you that The preceding equation comes from solving the radial Schrödinger equation. However the wave function above tells us nothing about where the particle is to be found in space.
Z kx ω. The solution is only good to a multiplicative constant so you add such a constant A nl which turns out to depend. E x t B kx t C kx t.
This guarantees that the wave function returns a single value for the probability for any state. The good news is that there is a simple formula for the wavenumber and you need only very basic information about the wave to calculate it. The Schrodinger Equation.
Requiring the wave function to terminate at the right end of the tube gives. The Schrodinger equation is linear partial differential equation that describes the evolution of a quantum state in a similar way to Newtons laws the second law in particular in classical mechanics. K 2π L 2π_f_ v.
A simpler equation for a harmonic wave. A 2 Integral from negative infinity to infinity dx e -a mx2h-bar 1. You could use sine if your wave started at this point and went up from there but ours start at a maximum so well use cosine.
E xt A. This means that the greater b is. Perhaps the simplest way to approximate the shape is with the sine function and inverse sine function.
All we get is probabilities. It is expressed as ψ x y z t a ib and the complex conjugate of the wave function is expressed as ψ x y z t a ib. A similar arrow below the probability density.
This may be a helpful definition but with the inclusion of a floor function it isnt graphable as-is. There are 4 main conditions that we need for a function to be a valid wave function. E xt A.
The Physical Significance of Wave Function. A stationary-state wave function contains no information about the position of the particles as a function of time. Ham Hvary_rangeiW evevc nplinalgeighham energiesappendev evecsappendevc Then find indices of energies near band crossing.
T sinθ which is the same result as before as long as. So even at points down here where the wave function has a negative value I mean you cant have a negative probability. Thus given the frequency and wave number of a wave function we can determine the speed of the particle from the phase velocity of its wave function v 2vp.
However the Schrodinger equation is a wave equation for the wave function of the particle in question and so the use of the equation to. The Wave Function PDF 4 Expectations Momentum and Uncertainty PDF 5 Operators and the Schrödinger Equation PDF 6 Time Evolution and the Schrödinger Equation PDF 7 More on Energy Eigenstates PDF 8 Quantum Harmonic Oscillator PDF. The smaller the period becomes.
T cosθ A. Beyond this interval the amplitude of the wave function is zero because the ball is confined to the tube. From knowing the total nodes we can find the number of radial nodes by using.
How do you calculate total nodes. S i n 3 x that the wave number is. Psi xt Ae -a mx2h-barit psi x0 Ae -a mx2h-bari 0 psi x0 Ae -a mx2h-bar Integral from negative infinity to infinity dx absolute valueAe -a mx2h-bar absolute value 2 1.
A wave function in quantum physics is a mathematical description of the quantum state of an isolated quantum systemThe wave function is a complex-valued probability amplitude and the probabilities for the possible results of measurements made on the system can be derived from itThe most common symbols for a wave function are the Greek letters ψ and Ψ lower-case. The wave function of an electron enables us to determine that probability. Instead it is complex.
An alternate definition is as the absolute value of the sawtooth wave but that definition also includes the floor function. The wave number b is illustrated here using the sine function defined by. So well say that our amplitude not just A our amplitude happens to be three meters because our water gets as high as three meters above the equilibrium level.
For example the following graph uses a. There is no physical meaning of wave function as it is not a quantity which can be observed. Energies evecs W 100for i in rangev_segs.
So the wave function does not tell you where the electrons gonna be. The wave function must be single valued. Psixt Aeikx-omega t Beikxomega t Which can create a stationary wave in some cases eg.
No comments for "How to Determine Which Wave Function to Use"
Post a Comment